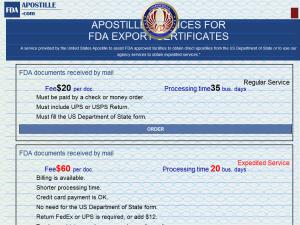
Apostille Services for FDA Export Certificates
Apostille Services for FDA Export Certificates
1615 Bay Head Rd #120
Annapolis, MD 21409
1615 Bay Head Rd #120
Annapolis, MD 21409
Foreign importers ask U.S. exporting companies to provide certification for products subject to the
Foreign importers ask U.S. exporting companies to provide certification for products subject to the Federal Food, Drug, and Cosmetic Act. FDA issues export certificates that contain information about the regulatory status of a particular product. FDA issues export certifications for drugs, biological products, and devices for humans and animals that meet the applicable requirements of the law and that can be legally marketed in the United States or that can be legally exported but not marketed in the United States. The FDA is not required to issue export certificates for food, feed additives, dietary supplements, or cosmetics. However, the FDA intends to continue to issue export certificates if resources permit.
Keywords: document legalization
Foreign importers ask U.S. exporting companies to provide certification for products subject to the Federal Food, Drug, and Cosmetic Act. FDA issues export certificates that contain information about the regulatory status of a particular product. FDA issues export certifications for drugs, biological products, and devices for humans and animals that meet the applicable requirements of the law and that can be legally marketed in the United States or that can be legally exported but not marketed in the United States. The FDA is not required to issue export certificates for food, feed additives, dietary supplements, or cosmetics. However, the FDA intends to continue to issue export certificates if resources permit.
Keywords: document legalization
Customer Reviews for Apostille Services for FDA Export Certificates
Be the first to review Apostille Services for FDA Export Certificates - Use the thumbs to get started!
Apostille Services for FDA Export Certificates has not yet completed their interview.